Human-Based Tissue Chip Technologies Predict Drug Effects in Humans Better Than Animals
The U.S. Food and Drug Administration generally requires new drugs to be tested for safety in animals before human clinical trials can be conducted.
However, when treatments pass such preclinical phases due to promising outcomes in animals, one-third fail in clinical trials because they are unsafe for humans, and two-thirds fail because they are ineffective. Overall, only about 10 percent of drug development programs successfully make it to market. This pattern of failure is bad for patients in need of safe, effective treatments, it is dangerous for clinical trial participants who experience negative outcomes, and it is resource-intensive.
It also highlights the need for more predictive preclinical testing approaches. Several large-scale studies investigating drug toxicity concordance between animals and humans have demonstrated that a lack of toxicity in preclinical animal tests is a poor predictor for the lack of adverse drug reactions in humans.
To address this need, the National Institutes of Health (NIH) launched the National Center for Advancing Translational Sciences in 2012 and began funding its Tissue Chip for Drug Screening program. This program aims to fund the development of tissue chips, miniature 3D platforms that accurately model the structure and function of human organs, in order to better model human diseases and to aid preclinical drug screening, safety, and efficacy testing. NIH has spent some $100 million on the program since its inception.
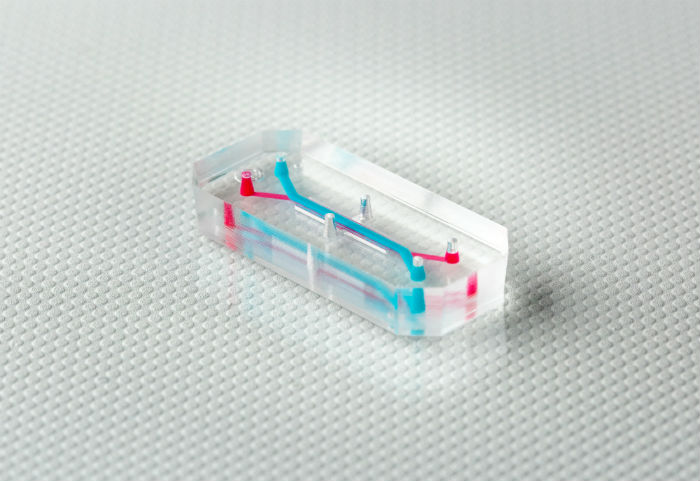
Tissue chips are tiny systems about the size of a USB stick consisting of human cells and microfluidic chambers capable of delivering nutrients and removing waste, embedded in transparent materials that allow real-time, longitudinal measurements to be taken. Importantly, cells can be arranged in 3D, allowing tissue chips to recapitulate complex cellular and tissue interactions that occur in the human body.
Tissue chips can be cultured with human cells derived from healthy and diseased donors in order to investigate the specific biological mechanisms of disease states. For example, researchers at the Wyss Institute have developed patient-derived tissue chips to study the kidney and lung dysfunction associated with kidney injury and respiratory disease, respectively. In addition, tissue chips can be cultured with multiple cell types to better evaluate unanticipated, off-target toxic effects, such as a three-organ tissue chip comprised of liver, heart, and lung and a five-chamber chip that can be reconfigured with a variety of healthy and cancer cell types, the latter of which we reviewed here.
In the coming years, tissue chip technologies may prove to be especially valuable in the study, diagnosis, and treatment of diseases in which an individual patient’s molecular pathology is important, such as rare diseases and cancer. Patient-derived tissue chips have the potential to become a predictive and cost-effective precision medicine approach for developing patient-specific therapeutics and determining patient-specific treatment strategies.
The tissue chip with perhaps the most immediate demand is the liver chip, such as the one being developed by scientists at Emulate, due to its applications for testing the liver toxicity of drugs and other compounds found in consumer products. A recent study comparing the toxicity of drugs in liver chips comprised of rat, dog, and human cells demonstrated significant differences in responses between the three species, reinforcing the need for human-based toxicology approaches. Human-based tissue chips are more predictive of human biology and align with ethical principles calling for the replacement of animals in research and testing.








